Maintaining your preclinical data management plan
You will know that you have a great data management plan when you barely have to think about data management anymore.
Imagine that you have already done the work of setting up a great data management plan. Your organization started by setting up a data governance board, and then together with Biolizard bridged the gaps between IT and R&D departments (covered in blog 1 in this series) devised a structured roadmap to implement a data management plan that suits your organization’s goals (outlined in the second blog in this series). You now have a group of people within your organization who are committed to making sure that your data management plan stays great – from the data stewards who do the day-to-day work of ensuring that standard operating procedures for data creation and management are followed, to the data champions who act as advisers, all the way to the chief data officer who oversees it all. It was a great joint effort, and now you get to see the benefits…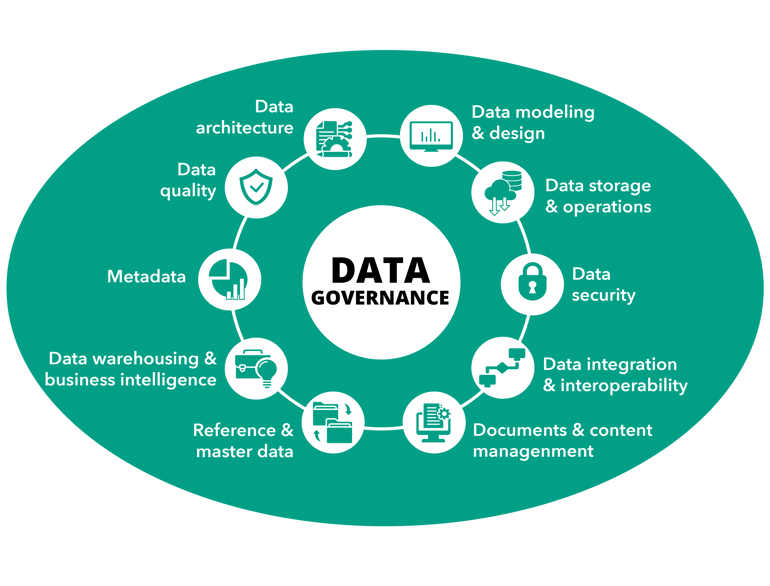
Image adapted from DAMA international (DAMA. Earley, S., & Henderson, D., Sebastian-Coleman, L (Eds.). The DAMA Guide to the Data Management Body of Knowledge (DAMA-DM BOK). Bradley Beach, NJ: Technics Publications, LLC. 2017.)
The benefits of great preclinical data management for an R&D scientist
As a scientist working in R&D, you’ll notice that having a good data management plan means that you have access to all of the insights that your data can provide, right at your fingertips. As well as having a simplified system to take a look at the latest data generated by you and your colleagues, you also can get real-time data insights with intuitive visuals. Taken together, you’ve noticed that this drastically cuts down on both meetings and repeat experiments because everybody has a much easier time staying aware of what everybody else is doing. No more data redundancy, hurray!
You have come to realise that you can think of data management as an iceberg. Before the implementation of your organization’s new-and-improved data management plan, a lot of that iceberg was above the water: there was so much work that you saw and had to do yourself, manually, to keep the iceberg from melting into a mess. But now that there is a good data management plan in place, that iceberg of data management has settled mostly below the water. Most tasks are now handled automatically by the systems put in place to manage them, and data management tasks no longer loom menacingly on the horizon of every workday. Finally, you can just focus on what you do best: biology!
The benefits of great preclinical data management for an IT professional
With the coming of your organization’s new data management plan, you’ve experienced a great sense of relief. Finally, you have a guarantee that all the internal and external rules of data compliance are followed on a daily basis. There is no more “shadow IT” – R&D no longer feels the need to set up their own mini IT systems that work for them but are not GDPR compliant, which they often did before the implementation of your organization’s new and upgraded data management plan. You can also see that you have much happier ‘customers’ in the R&D team. There are fewer complaints about the IT system and far fewer urgent requests.
Now, when a new project begins, there’s no time wasted on gathering additional context about the biological data from the R&D team. All of the new data is automatically easy to manage because it’s FAIR from the get-go. Thanks to the new and improved data management plan, business operations are running much smoother and you no longer feel like a computational firefighter – meaning that you can focus on the more interesting things…
For instance, you know that having a great data management plan and data operations system in place is a prerequisite for implementing AI approaches. You can now see more clearly how AI could be applied to create brand-new insights based on the data inputted by R&D. And, since the new data management system has cut costs by boosting employee efficiency, reducing the unnecessary generation of data, and cutting out unneeded cloud storage, there is finally the budget to start implementing more high-tech tools like machine learning and AI. It’s an exciting time!
Maintaining your preclinical data management plan as your business grows
You can think of your organization’s data like a living, changing asset: the type of data that you’re inputting may change, the insights required from the same data types may be altered, or the organization’s business goals and the data required to meet them may be modified over time. That’s why, although the bulk of the time and monetary investment in data management is made upfront in creating a great data management plan, maintaining that plan is still a continuous investment.
When BioLizard partners with organizations to build their perfect data management plan, we always plan for future efficiency and success in its maintenance. To accomplish this, we train internal data stewards within the company to do the ‘aftercare’ of complying with the agreements set out in a data management roadmap. This means that as your organization grows, it will be possible to seamlessly scale and integrate new data without constantly needing to rework your plan for data management.
However, when you are making a major change, such as starting to work with an entirely new data type, it is important to revisit and adjust your data management plan so that it can continue to work for you. This will make sure that the majority of your data management iceberg stays underwater, and under control!
Do you need help adjusting your data management plan to suit your new needs?
Reach out to BioLizard!






